Take-home point: Rule out lithium toxicity in all patients on lithium presenting with cardiac toxicity and/or EKG changes.
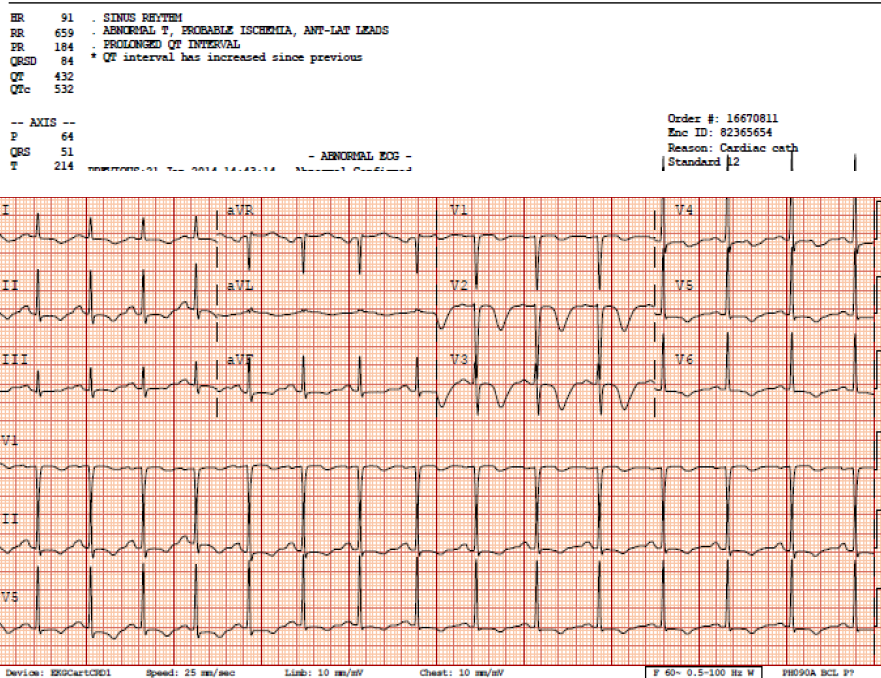
[1] The case: 48 yo F with history of bipolar disease and depression presents to ED for evaluation of substernal chest pressure radiating to neck with bilateral arm "numbness." Mild shortness of breath and ROS only positive for mild gradual onset headache. Symptoms x 7 hours. Chest pain non-exertional, non-positional, and non-pleuritic. No fever, focal neurologic deficits, mental status changes, or abdominal pain. Chest pain 7/10 upon arrival, and improved with 3/10 with nitrate therapy but continued to have ongoing pain/symptoms. Serial EKG revealed dynamic change, and concern for proximal LAD lesion and Wellen's syndrome was discussed with cardiology. Cardiology performed a bedside echo which revealed anterior wall motion abnormality, and to a lesser degree inferior-lateral dyskinesia. Patient was taken to cath lab for elective cath, with knowledge of an elevated troponin of 0.61 (normal < 0.03), Cardiac cath revealed completely normal coronaries, and no LV aneurysm Lithium level came back elevated at 2.3, with no history of acute ingestion. Quick literature review (articles attached) revealed that lithium can mimic ischemic EKGs, especially in the anterior leads. Further, chronic lithium toxicity can also cause myocarditis. See EKGs below illustrating deep anterior T wave inversions and prolonged QTc, both of which resolved after lithium level decreased.
[2] Quick skinny on lithium toxicity and EKGs, attached is are 2 review articles as well.
- Lithium causes toxicity through several mechanisms including competition with many electrolytes (sodium, potassium, calcium, and magnesium) and ion transporters, which can disrupt many cellular functions. The cellular membrane of concern here is myocytes (and Purkinje fibers....and most people just closed this email!), and these disruptions manifest as EKG changes.
- Common EKG changes include [a] QTs prolongation, [b] nonspecific ST segment and T wave abnormalities, [c] T wave inversions (reference #1), [d] transient ST elevations mimicking acute myocardial infarction (reference #2), [e] Brugada changes with possibility of sudden cardiac death (reference #3), [f] SA nodal dysfunction and heart block, and [g] severe bradycardia.
- ECG changes are more likely to occur with chronic lithium overdoses than acute overdoses
- Treatment is beyond the scope of this review but some important things to consider are
- Make sure you get lithium levels on all patients presenting with cardiac symptoms
- Know the difference between acute vs chronic toxicity, read attached article
- Know your criteria for hemodialysis
- Avoid thiazides and ACE-I, as these will increase lithium levels
- Avoid NSAIDS, can also worsen toxicity. Often given for myocarditis, but do not given if you think this is lithium-induced myocarditis
- Check TSH, can be contributing to lithium toxicity
- Consult toxicology and nephrology early, especially if cardiology is going to manage primarily
Feedback always welcome (joshua.eastvold@gmail.com),
Also, if anyone is interested in publishing this let me know??
Josh
References:
[1] Cases J. 2008 Sep 17;1(1):156. doi: 10.1186/1757-1626-1-156.
[2] J Med Toxicol. 2008 Sep;4(3):170-2.
[3] Circulation. 2010 Aug 10;122(6):e418-9
[4] Toxicol Rev. 2006;25(4):221-30.
[5] J Am Soc Nephrol. 1999 Mar;10(3):666-74
EKG #1 upon arrival to ED
EKG #2 - concerning for anterior ischemic. QTc 494.
EKG #3 - worsening QTc prolongation, still getting PO lithium, don't ask
EKG #4 - Courtesy call to cardiology, relayed my thoughts regarding concern for chronic lithium toxicity
-- now after lithium level returned to 1.3, complete resolution of anterior T wave changes and normalization of QTc